How soil fungal communities respond to invasive plant species treatments in soil from Banksia woodland, south-western Australia
Aaron J. Brace A B * , Katinka X. Ruthrof B C , Joseph B. Fontaine C , Ben P. Miller
A B * , Katinka X. Ruthrof B C , Joseph B. Fontaine C , Ben P. Miller  D and Anna J. M. Hopkins A E
D and Anna J. M. Hopkins A E
A
B
C
D
E
Abstract
Invasive plants are one of the most significant threats to woodlands globally. Methods of invasive plant control include manual removal and herbicide application. While the impacts of control methods on invasive and off-target native plant species are often explored, the impacts on below-ground organisms, such as fungi, are less well understood.
We conducted a glasshouse trial to investigate the responses of soil fungal communities to herbicides and manual removal that are used to control common invasive plant species in Banksia woodland in south-western Australia.
Broad spectrum (glyphosate and pelargonic acid) and grass-specific (fluazifop-p-butyl) herbicides were separately applied to pots containing either Ehrharta calycina, a key invasive grass species or Eucalyptus todtiana, a native woodland tree at the recommended woodland rate. After six weeks, samples of treated soils were subjected to high throughput sequencing to determine fungal community diversity, richness, relative abundance, composition and putative ecosystem function.
Pelargonic acid induced the widest range of changes including decreased fungal richness and Shannon diversity but all herbicides affected community composition. Within functional groups, fluazifop-p-butyl led to a significant decrease of symbiotrophs in the mycorrhizal species.
We show that invasive species management, in the manner applied, can lead to immediate changes in fungal community composition.
Observed patterns require further exploration, particularly repeat testing under different environmental conditions, to better determine the impact and mode of action of herbicides on below-ground organisms. The functional changes in the soil fungal community could further disturb the soil fungal community and complicate subsequent management considerations.
Keywords: bioinformatics, eDNA, herbicide effects, invasive species management, ITS subregion, Mediterranean type climate ecosystem, non-target damage, soil fungi.
Introduction
After land clearing, invasive species are the most significant global threat to biodiversity (Clements et al. 2004; Zedler and Kercher 2004; Paini et al. 2016). Herbicides used to control invasive plant species can include many modes of biochemical action, and specialised mixtures, techniques, and application timing and methods depending on the target (Rüegg et al. 2007; He et al. 2022; Pranaswi et al. 2022). However, with the widespread and increasing use of herbicides in native ecosystems to control invasive species, more attention is being paid to the non-target damage and environmental toxicity (Kudsk 2008; Van Bruggen et al. 2018; Giglio and Vommaro 2022). Generally, herbicides are classified as either broad spectrum or target-specific. Broad spectrum herbicides (e.g. those containing glyphosate N-[phosphonomethyl]glycine) work by affecting metabolic processes such as the shikimic pathway, a step required for protein synthesis in all plants (Helander et al. 2012). Target-specific herbicides, such as those containing fluazifop-p-butyl work by inhibiting specific enzymes used in fatty acid synthesis that are only found in members of the Poaceae (Walker et al. 1988). For many herbicides, application and integration into woodland management has been occurring since the 1950s, with incremental developments adding to the usage and biochemical action. For example, glyphosate-based herbicides were first introduced in the 1970s but the formulation has been updated over time (Grossmann 2010; Duke 2018). Less is known about target impacts for some more recently introduced herbicides. These include herbicides based on pelargonic acid and synthesised from esters in the oil of Pelargonium spp. that lead to necrotic lesions on surface contact (Ciriminna et al. 2019). Although the precise pathway is not known, studies have shown that pelargonic acid is a viable alternative to more common synthetic herbicides (Loddo et al. 2023). However, whether long term persistence and non-target toxicity is suitable for wider ecosystem use is unclear (Muñoz et al. 2022).
Increasingly, concerns have been raised about the impacts of herbicide application on different non-target trophic levels from decomposers (soil microorganisms) to primary consumers (insect herbivores) all the way up to tertiary consumers (predatory fish) (Pratt et al. 1997; Relyea 2005; Russell and Schultz 2010; Guilherme et al. 2012). Soil microorganisms, important to ecosystem function and plant community persistence but difficult to study, have been the subject of a few studies examining the impacts of herbicides (Roca et al. 2009; Zaller et al. 2014; Rose et al. 2016; Lekberg et al. 2017). Understanding the impacts of herbicides on non-target organisms such as soil fungi is important, as fungal communities are integral to ecosystem functions such as nutrient cycling, primary productivity, ecosystem health and competition dynamics (Tedersoo et al. 2014).
Soil fungi can be divided into three broad groups according to the functional roles fulfilled, namely saprotrophs, mutualists and pathogens. Saprotrophs play a critical role in the cycling of carbon and nitrogen through the decomposition of organic matter in the soil and litter, making these a highly significant functional group of fungi. Cellulose, hemicellulose and lignin are the primary carbon and nitrogen sources that accumulate within woody debris in forest ecosystems (Laiho and Prescott 1999; Amundson 2001). Herbicides can immobilise nutrient cycles in these fungi. For example, a dinitroaniline-based grass selective herbicide applied to ten saprotrophic basidiomycetes isolated from soil in southern Italy showed a decrease in mycelial growth that was also correlated with increased herbicide concentration (Roca et al. 2009). Glyphosate-based herbicides have also been shown to have fungicidal properties on saprotrophic fungi (Chávez-Ortiz et al. 2022).
As mutualists, fungi can provide a variety of benefits to the host plant such as improved disease resistance, heavy metal tolerance, higher drought resistance, better soil structure and improved nutrient uptake (Gosling et al. 2006). Therefore, if these fungi were to be affected by external factors (such as invasive species management) these benefits could be diminished. The effects of herbicides on mutualist fungi range widely. For example, application of simazine-based herbicide (broad spectrum, surface-applied) in Pinus spp. plantations resulted in no inhibition of ectomycorrhiza and at some application rates even enhanced the growth of mycorrhiza (Uhlig 1966; Smith and Ferry 1979). Studies also indicate that a variety of herbicides negatively impact mutualistic fungi (Singh et al. 2020). Application of herbicides with the active ingredients prometryn, chloroacetanilide or nicosulfuron (for use as foliar broad spectrum herbicides) all showed a decrease in mycorrhizal growth and associations made in glasshouse trials, independent from impact on host species (Li et al. 2013; Karpouzas et al. 2014). Other studies, however, have shown minimal effects of herbicides on mycorrhizal species (Rose et al. 2016). Noting that many of these effects may be a direct result of the herbicide but could also be indirect effects from changes in the environment (Edwards and Pimentel 1989; Lekberg et al. 2017) such as mortality of target plants is important. Pathogenic fungi are receiving increasing attention, with more focus on the spread, infection and virulence mechanisns to better inform management practices. There have been many studies in cropping systems of herbicide effects on fungal pathogens but a variety of effects have been observed, both in increasing and reducing relative abundance of pathogenic fungi (Sanyal 2008; Leino et al. 2021). Understanding the direct and indirect impacts of herbicides on these fungal functional groups will help inform management and conservation decisions regarding natural ecosystems.
One method of disentangling direct from indirect effects of herbicide on the soil fungal community is to test whether the effects of chemical death on plants induced by herbicides are the same as those by mechanical mortality. Mechanical removal, conducted by hand, can have minimal impacts, however, this can be very expensive and time consuming (Zhao et al. 2011). One type of mechanical removal, ploughing (overturning the top ~30 cm of soil), has been shown to significantly decrease the soil microbial community biomass and richness (Schnoor et al. 2011).
There are clearly many responses to explore regarding the interaction between soil fungal communities and herbicides used in native ecosystem management and restoration. Herbicides such as those based on glyphosate, fluazifop-p-butyl and pelargonic acid are widely used to manage invasive plants. A study on these herbicides used in conjunction with prescribed burns to control invasive species showed that herbicide alone had the most deleterious effects in two urban Banksia woodlands in south-western Australia (Brace et al. 2025).
To further assess the impact of herbicides on soil fungal communities in the 128 Banksia woodlands, we conducted a glasshouse trial to isolate fungal responses from other environmental influences. We examined herbicide application on soil associated with a widespread problem weed, South African perennial veldt grass (Ehrharta calycina) that we contrasted with pricklybark, a native eucalypt tree (Eucalyptus todtiana) and empty pots (containing soil but no vegetation). In addition to using the three herbicides, we implemented a mechanical clipping treatment, enabling us to distinguish herbicide effects from those from plant death on the soil community. Our fully factorial glasshouse experiment quantified soil fungal community responses (composition, richness and diversity) and changes in relative abundance of taxonomic (phyla, families) and functional groups, and we aim to test three hypotheses:
−Herbicide application will induce declines in richness, diversity and relative abundance of soil fungi compared to the control pots.
−Mechanical plant mortality will induce a different response on soil fungi from chemically-induced plant mortality.
−The soil fungal community in pots containing the native tree species that is known to associate with local fungi will be more adversely affected than the soil fungal community in pots containing the invasive grass species.
Materials and methods
Study site region
The soils for this ex situ, glasshouse-based study were collected from Kings Park, a 267-ha urban woodland, located 1–4 km from the centre of Western Australia’s state capital (Perth) on the Swan Coastal Plain in south-western Australia. The Mediterranean climate of the region is characterised as temperate, featuring hot, dry summers and cool, wet winters (Kottek et al. 2006; Peel et al. 2007). Perth’s mean annual rainfall was 735 mm for the period 1992–2022, most of which falls in April–November. In 2020 (the year of soil collection), the summer (December–February) mean daily maximum temperature was 32.0°C and the winter (June–August), 19.9°C (Perth Metro weather station; Australian Government Bureau of Meteorology 2023). Kings Park is located on the Spearwood dune system that consists of deep, well-draining, low nutrient sands over limestone parent material that has existed in this state for ~120,000–500,000 years (McArthur and Bettenay 1960; McArthur and Bartle 1980). This soil type is widespread in the Perth urban metropolitan area in which many urban bushland remnants require weed management.
Study species
For the glasshouse trial, two species were selected: the invasive grass Ehrharta calycina (Poaceae) and native tree Eucalyptus todtiana (Myrtaceae). Ehrharta calycina is an established, problematic perennial grassy weed in Australia that is spreading and poses a threat to local biodiversity (Fisher et al. 2009; Keighery et al. 2023). Eucalyptus todtiana is widely distributed, provides a key structural component of ecosystems of the Swan Coastal Plain and is a member of a widespread and ecologically significant genus (Ritchie et al. 2021).
Experimental design
To assess the impact of herbicides on soil fungi, we implemented a 20-week glasshouse-based experiment. Soil for this glasshouse study was collected under permit from an area in Kings Park not previously treated with herbicides. Sampling occurred in March 2020 via the digging of five pits (~20 m apart, litter removed first) and collecting soil from the top 20 cm of the soil profile (1 kg per pit) with a shovel. The shovel was sterilised with 80% sodium hypochlorite between pits. Soil samples were combined and placed into new, sealable food-safe plastic bags, put on ice and immediately taken to be stored in a −20° freezer. To provide adequate replication for treatments the soil samples were subsequently combined with sterilised white and yellow coarse sand (Yates et al. 2016) in a 1:10 ratio to provide an ‘inoculum’ of soil biota from the Kings Park soils. This mixed soil was placed into new, free draining, 140 mm (1.3 L) pots (Sunpalm Australia, Wangara, Western Australia). Pots were sterilised with 80% sodium hypochlorite and had a fresh 200 mm2 absorbent cloth placed into the bottom of the pot to prevent sand draining from pots. One kilogram of soil mixture was placed on top of the cloth in each pot.
Ehrharta calycina seeds were collected from Kings Park by staff and volunteers in early 2016. Eucalyptus todtiana seeds were sourced from a local seed merchant (Nindethana, King River, Western Australia), collected from a wild population in Banksia woodland near Lancelin, 120 km north of Kings Park, Western Australia in 2018. All seeds were X-rayed (at Kings Park laboratories, Department of Biodiversity Conservation and Attractions, Perth, Western Australia – utilising a Faxitron Specimen Radiography System, Marlborough, Massachusetts, USA) to assess viability and surface sterilised (with 50% sodium hypochlorite; Lindsey III et al. 2017). Pots were sown with 40 seeds, 20 at the surface and 20 at 5 mm depth, to maximise germination likelihood. If multiple seedlings germinated, these were thinned, allowing only one individual to remain per pot. This totalled N = 126 pots: 45 pots containing Ehrharta calycina, 45 containing Eucalyptus todtiana and 36 containing bare soil. These were split into five treatments: glyphosate, fluazifop-p-butyl, pelargonic acid, a mechanical removal treatment (by cutting off stems at the base with sterile secateurs) and no treatment, resulting in nine replicates per vegetation type per treatment. The mechanical treatment, and herbicide applied to bare pots, aimed to disentangle the mortality of the plant from the effects of the herbicide (Fig. 1).
The treatments, timing and species used to observe the effects of herbicides on the soil fungal community in pots that were bare or planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia. The species management strategies included no strategy, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid. There was a total of 126 pots: 45 pots each of the invasive grass, Ehrharta calycina and the native tree, Eucalyptus todtiana, across five treatments and 36 bare pots across four treatments (no mechanical removal).

Once seeds were sown, pots were watered automatically by an irrigation system: 1 min/2 times a day for 14 weeks from January to May 2021 in glasshouses at Edith Cowan University, Joondalup, Western Australia. After the 14-week growing period, herbicide and mechanical removal treatments were applied. Herbicides treatments were applied in May 2021 (autumn) by a PA6 trained technician via a pump sprayer hand actuated to ~40 psi with a fan nozzle at recommended rates for control in natural areas (Table 1).
| Active ingredient | Mode of action | Specificity | Recommended/active concentration (gL-1) | Application rate (woodland) | |
|---|---|---|---|---|---|
| Glyphosate acid and related salts | Disruption of the shikimate acid pathway A | Broad spectrum, non-specific | 450 | 10 mL/L (spot spray until wet) | |
| Fluazifop-p-butyl | Prevents fatty acid synthesis B | Grass specific | 128 | 3.3–6.6 L/ha (density dependent) | |
| Pelargonic acid | Disrupts pH within cells causing localised cell death C | Broad spectrum, non-specific | 525 | 70 mL/L (spot spray until wet) |
Pots were treated with different invasive species management strategies (none, mechanical removal, glyphosate, fluazifop-p-butyl and pelargonic acid).
All individuals (of both the invasive grass and native tree) subjected to herbicide treatments had complete mortality within the first 24 h. Six weeks after herbicide application, in June 2021, seedlings were destructively harvested with sterile equipment and a subsample of the soil was collected from each pot for molecular analysis. Soil was collected with 25 mm wide corers pushed 120 mm deep four times within each pot (adapted from Yates et al. 2016; Ritchie et al. 2020), bulked and placed into clean, labelled plastic bags. Corers were sterilised with 80% sodium hypochlorite between each pot and samples were frozen at −20°C until analysis.
DNA extraction
Soil core samples from each pot were homogenised and sieved (2 mm pore size; sieve was sterilised with 80% sodium hypochlorite between samples) to remove any debris or large aggregates. Subsamples of 250 mg were taken and DNA was extracted using a Qiagen DNeasy PowerSoil Pro DNA kit following the manufacturers protocol. Amplification was conducted using a primer combination specific for higher fungi: fITS7 and ITS4 (White et al. 1990; Ihrmark et al. 2012). PCR amplification and sequencing were undertaken as described in Brace et al. (2024). In brief, a 25 μL mixture with primers targeting the fITS7 (TACGGTAGCAGAGACTTGGTCTGGGTGARTCATCGAATCTTTG) and ITS4 (ACACTGACGACATGGTTCTACACGCCTSCSCTTANTDATATGC) region (White et al. 1990; Ihrmark et al. 2012), at a 57°C annealing temperature in a QuantStudio™ 5 Real-Time PCR system, and sequencing undertaken on an Illumina MiSeq at the TrEnD laboratories at Curtin University, Bentley, Western Australia. No sequencing reads were present in either the sample extraction controls or PCR controls.
Bioinformatics
Fungal ITS sequences were initially demultiplexed using the insect package (Wilkinson et al. 2018) and subsequently processed using the DADA2 bioinformatics (Callahan et al. 2016) pipeline in R 4.1.0 programming language (R Core Team 2020) and RStudio ver. 4.1.0 (R Studio Team 2021). The sequences underwent trimming, filtering and quality control according to the visual inspection of read quality profiles. Merged pairs with a length below 50 base pairs were excluded. Sequence primers were removed using the cutadapt tool (Martin 2011) in RStudio. The remaining sequences were clustered at the nucleotide level to identify amplicon sequence variants (ASVs). Bimeric, chimeric and singleton ASVs were eliminated using the ‘nochim’ function following the DADA2 methodology (Callahan et al. 2016).
ASVs were assigned putative taxonomy based on the closest match found in the custom curated Unite ver. 9.0 database for fungi (Abarenkov et al. 2010), utilising naïve Bayesian classification of minBoot bootstrap confidence levels with the ‘assignTaxonomy’ function in DADA2. Reads that mapped to these reference databases and were identifiable at least at the Kingdom level were retained. Reads that could not be identified at the Kingdom level or were identified as belonging to a different Kingdom were considered unknown and excluded from further analyses. Fungal ITS ASVs were categorised based on putative life history using ecological guild assignment as described by FUNGuild in Nguyen et al. (2016). The guilds used in this study (at probable or above) were symbiotrophs, saprotrophs and pathotrophs. Amplicon Sequence Variants with multifaceted guild membership were allocated to both relevant groups and those without a function were counted as unknown in further analysis.
The sequence table and taxonomic assignment data obtained from DADA2 were merged with a sample matrix using the phyloseq package (McMurdie and Holmes 2013) to enable comparisons at the site and sample level. Using the ‘prune_taxa’ tool in phyloseq Amplicon Sequence Variants with more than five reads in at least 5% of the samples were retained for statistical analyses (Brace et al. 2024).
Data analysis
The main goals of this study were to quantify changes in soil fungal community composition and relative abundance of taxonomic and functional groups in relation to vegetation type (empty, invasive grass, native tree) and pot treatment (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid).
To reveal the effect of treatment and pot occupant on the soil fungal community, Shannon diversity and observed species richness of Amplified Sequence Variants (ASVs) were calculated using the ‘estimate_richness’ function in phyloseq. To examine compositional shifts in the fungal community in these different treatment and pot occupant combinations, a non-metric dimensional scaling (NMDS) analysis based on Bray–Curtis dissimilarity was conducted utilising the ‘vegdist’ function from the vegan package (Oksanen et al. 2017) using relative abundance data derived from the ASV species matrix. Additionally, a permutational multivariate analysis of variance (PERMANOVA) was used to quantify difference in community composition among various groups. The PERMANOVA was executed through the vegan package (Dixon 2003) utilising the ‘adonis’ function with 999 permutations. This analysis evaluated variation in community composition across treatment and pot occupant combinations.
Generalised mixed-effect models were used to test for the effects of herbicide application on the soil fungal community. These generalised mixed-models were run utilising the glmmTMB package (Brooks et al. 2017) and visually inspecting residuals with the DHARMa package (Hartig and Hartig 2017). Utilising Gaussian error distribution, vegetation type and treatment were fixed effects and to account for repeat measures, were used as a random effect. Significant effects were those with a P-value of less than 0.05 and for each significant result we report model estimate, standard error (s.e.), test statistic (z) and P-value, and provide full model outputs and residual distributions in the supplementary material (Supplementary Figs S1 and S2).
To identify fungal phyla, families and functional groupings, the abundance of which differed across treatments, analysis of composition of microbiomes with bias correction was utilised (ANCOMBC; Lin et al. 2021), as this is the most robust method in dealing with sequencing depth and inherent compositionality of datasets such as ours (Swift et al. 2023). The ANCOMBC method assumes that the observed abundance (false negatives – taxa present in the environment but not observed due to sequencing depth) of a given grouping is proportional to unobserved abundances per sample. The unobserved abundance sampling fraction is accounted for by an offset term (in this example ‘repeat’ is used) in a linear regression model (along with pot occupant and treatment being fixed terms). The ANCOMBC method dealt with the compositional nature of the dataset and was able to identify true structural zeroes and bias-correct false discover rate. This included P-value correction using the Holm-Bonferroni method for multiple samples. Log fold change, wherein observed counts are log transformed with the addition of zero counts to provide a metric of differential abundance are chosen to represent a change in relative abundance (Lin and Peddada 2020).
Analyses were also undertaken to establish whether any phyla, family or functional groups were particularly associated with a pot occupant or treatment via an indicator species analysis (Dufrêne and Legendre 1997). This methodology is based upon fidelity and exclusivity, and provides an indicator value (IV) based on whether a species is present in a singular site or present in all sites.
All data visualisation was undertaken with ggplot2 (Wickham 2011). For diversity metrics we report means and 95% confidence intervals, interpreting the visual non-overlap of confidence intervals with other group means as an indicator of statistical significance to be confirmed with testing (Ramsey and Schafer 2012). For visualisation of ANCOMBC modelling, we provide log fold change and standard error estimates.
Results
Soil fungal community
Across all pots in the glasshouse experiment, 230,159 sequences were obtained, resulting in 234 ASVs, of which 96% were identified to phylum, 94% to family and 68% to genus. Observed ASV richness was significantly different under treatments and differed between pot occupants (Fig. 2a). Richness was higher in pots containing the invasive grass, Ehrharta calycina and the native tree, Eucalyptus todtiana than in the bare pots (Generalised linear mixed model; Estimate = 9.13, s.e. = 3.17, z = 2.88, P = 0.004 and Estimate = 10.86, s.e. = 3.15, z = 3.44, P = 0.001 respectively). Overall, pots treated with mechanical removal and pelargonic acid were the only treatments to induce a significant decrease in richness compared to no treatment (Estimate = −11.68, s.e. = 4.35, z = −2.69, P = 0.007 and Estimate = −8.34, s.e. = 3.77, z = −2.21, P = 0.027 respectively). When comparing pots treated with mechanical removal to the other treatments, richness in pots treated with fluazifop-p-butyl pots was significantly higher (Estimate = 10.03, s.e. = 3.17, z = 3.16, P = 0.002; Fig. 2a, Supplementary Table S1). Shannon diversity index was significantly higher in pots containing the invasive grass Ehrharta calycina than in the bare pots and those with the native tree Eucalyptus todtiana (Estimate = 0.36, s.e. = 0.18, z = 2.03, P = 0.042). Shannon diversity of fungi in pots treated with pelargonic acid (Estimate = −0.54, s.e. = 0.21, z = −2.56, P = 0.010) and mechanical application (Estimate = −0.49, s.e. = 0.24, z = −2.03, P = 0.042) were significantly lower overall than the control pots. When comparing pots treated with mechanical removal to the other treatments, diversity in pots treated with glyphosate and fluazifop-p-butyl pots was significantly higher (Estimate = 0.48, s.e. = 0.24, z = 2.01, P = 0.044, and Estimate = 0.71, s.e. = 0.24, z = 2.95, P = 0.003, respectively; Fig. 2b, Table S1).
Mean ASV richness (a) and Mean Shannon diversity index (b) in pots that were bare or planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia, under different invasive species management strategies (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid). Plotted are Means and 95% confidence intervals are plotted, and letters indicate significant differences.

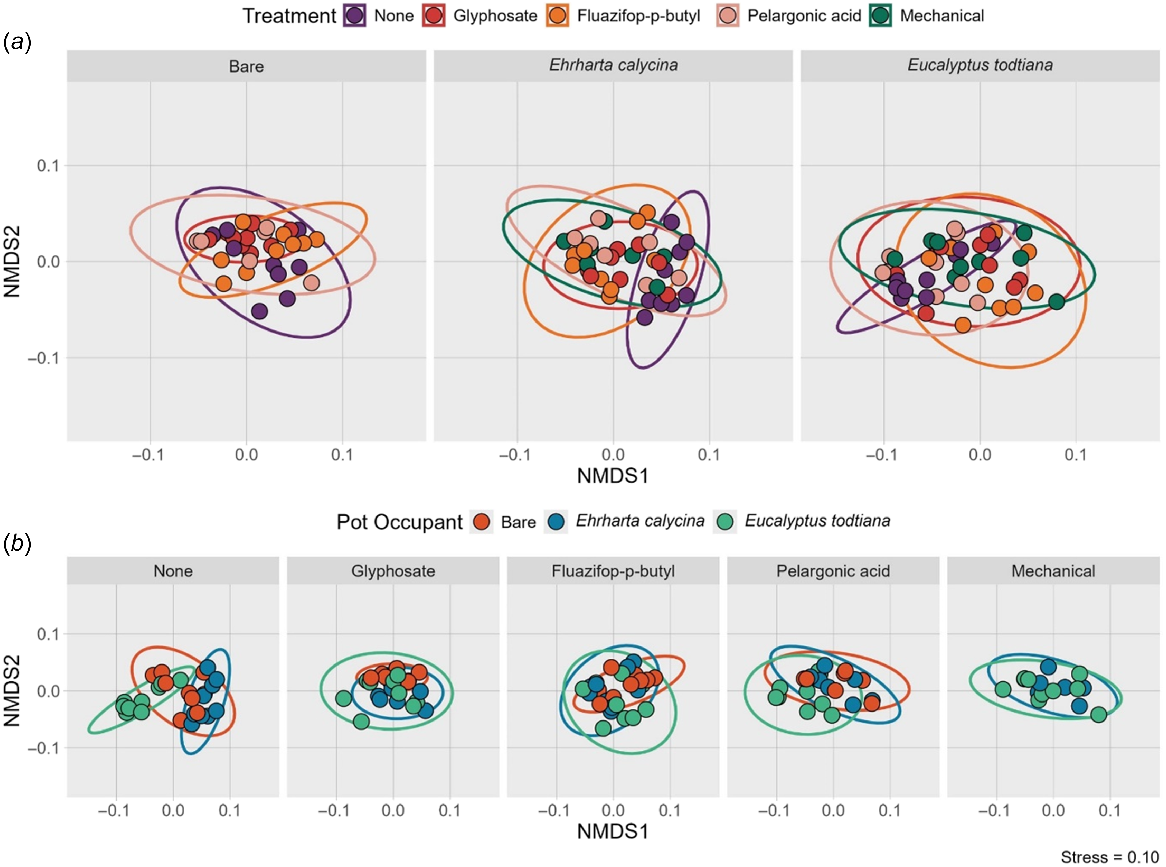
The NMDS matrices of ASV by sample had a two-dimensional solution with a stress of 0.10. Community composition was not significantly different between the three types of pot occupants (PERMANOVA: R2:0.01, P = 0.576, R2:0.01, P = 0.346, and R2:0.01, P = 0.906, respectively) but there were significant treatment differences in community composition. In the bare pots both those treated with glyphosate and fluazifop-p-butyl were significantly different to no treatment (R2:0.08, P = 0.001 and R2: 0.08, P = 0.005, respectively). In pots containing the invasive grass Ehrharta calycina the pots treated with glyphosate application had a significantly different community composition from the pots without treatment (R2:0.08, P = 0.050). There were no significant differences in community composition under different treatments in the native tree Eucalyptus todtiana (Fig. 3).
Two-dimensional NMDS Bray–Curtis dissimilarity plot of the soil fungal community composition of different fungi in pots that were bare, and pots that were planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia, under different invasive species management strategies (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid). Colours in panel (a) indicate treatments and colours in panel (b) indicate pot occupant, while ellipses represent 95% confidence intervals.
Abundances
Through the indicator species analysis, there were four (1.7%) ASVs that had significantly higher incidence with a specific pot occupant, all associated with the bare pots (Fig. 4). There were 18 ASVs (7.6%) associated with only one treatment, two (0.9%) with pots treated with glyphosate, two (0.9%) with pots treated with fluazifop-p-butyl and 14 (6%) with pots treated with mechanical removal. Phylum level community composition showed significantly different compositional abundance by treatment and pot occupant (Fig. 4). Through the ANCOMBC analysis of the seven phyla analysed, two showed a response to pot occupant and two showed a treatment response (all increases; Fig. 4b, Table S2).
(a) Phylogenetic classification of the fungal communities at the phylum level in pots that were bare, and pots that were planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia, under different invasive species management strategies (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid). There was a total of 126 pots, including 45 pots containing the invasive grass Ehrharta calycina and 45 pots containing the native tree Eucalyptus todtiana across five treatments, and 36 bare pots across four treatments (no mechanical removal). Plots show proportional averages. (b) Phyla that ANCOMBC denoted as having a significant difference in abundance. Plots show log fold change and standard error derived from model results (Table S2). Colours are shared with plot A.

Through the ANCOMBC analysis of the 50 families, six were identified to be significantly different by pot occupant (two higher in both Ehrharta calycina and Eucalyptus todtiana, and two lower in Eucalyptus todtiana) and six were identified to have a significant response to a treatment (five positive, one negative; Fig. 5b, Table S3).
(a) Phylogenetic classification of the fungal communities at the family level in pots that were bare, and pots that were planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia, under different invasive species management strategies (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid). There was a total of 126 pots, including 45 pots with the invasive grass Ehrharta calycina and 45 pots with the native tree Eucalyptus todtiana across five treatments, and 36 bare pots across four treatments (no mechanical removal). Proportional averages are plotted. (b) Families that ANCOMBC denoted as having a significant difference in abundance, plots show log fold change and standard error derived from model results (Table S2). Colours are shared with plot A.

At the family level, certain taxonomic groups were more strongly associated with specific pot occupants and treatments: Agaricaceae with bare pots (Indicator species analysis; IV = 0.14, P = 0.04), Sporormiaceae and Trichomeriaceae with the invasive grass Ehrharta calycina (IV = 0.25, P = 0.02, and IV = 0.17, P = 0.02) and Bulleribasidiaceae with the native tree Eucalyptus todtiana (IV = 0.19, P = 0.02). Associations were also observed in the treatment groups, with Orbiliaceae and Lyophyllaceae significantly correlated with fluazifop-p-butyl (IV = 0.39, P =< 0.01 and IV = 0.29, P = 0.03 respectively), Pleosporaceae correlated with glyphosate treatments (IV = 0.31, P =< 0.01) and Powellomycetaceae with mechanical removal treatments (IV = 0.30, P = 0.02; Fig. 5a).
Fungal functional groups had significantly different compositional abundance by pot occupant and treatment (Fig. 6a). Through the ANCOMBC analysis of the four functional groups, symbiotrophs were identified to be significantly more abundant in the Eucalyptus todtiana pots and seven were affected significantly by a treatment. Pots containing Ehrharta calycina treated with glyphosate had a significantly higher relative abundance of fungi with unknown function, pots treated with fluazifop-p-butyl had a significantly higher relative abundance of pathotrophs and pots treated mechanically had a significantly lower relative abundance than the pots with no treatment. In pots containing Ehrharta calycina the mechanical treatment also yielded a significantly lower relative abundance of saprotrophs in the soil and those with unknown function than glyphosate and significantly lower relative abundance of saprotrophs than fluazifop-p-butyl. In pots containing Eucalyptus todtiana, those treated with fluazifop-p-butyl had significantly lower relative abundance of symbiotrophs than pots that had no treatment (Fig. 6b, Table S4).
(a) Functional groups of the fungal communities in pots that were bare, and pots that were planted with the invasive grass Ehrharta calycina and the native tree Eucalyptus todtiana from south-western Australia, under different invasive species management strategies (none, mechanical removal, and application of glyphosate, fluazifop-p-butyl and pelargonic acid). There was a total of 126 pots, including 45 pots containing the invasive grass Ehrharta calycina and 45 pots containing the native tree Eucalyptus todtiana across five treatments, and 36 bare pots across four treatments (no mechanical removal). Plots show proportional averages. (b) Functional groups that ANCOMBC denoted as having a significant difference in abundance. The log fold change and standard error derived from model results (Table S2) are plotted. Colours are shared in plot A.

The indicator species analysis, when grouped by functional group, showed no significant associations to any pot occupant or treatment.
Discussion
This study has shown that invasive species management can alter the soil fungal community of Banksia woodland soil under ex situ conditions. Whilst these findings come from glasshouse conditions, we were able to study the effect of treatments without any confounding environmental variables. Brace et al. (2025) found similar effects in situ. Over 250 ASV taxa were identified and effects of the four invasive species treatments examined were contingent on pot occupants. The application of pelargonic acid and the mechanical treatment had the most significant effects (four and two respectively) on observed fungal ASV richness and Shannon’s diversity, leading to significant decreases compared to the untreated control, however pelargonic acid had the least effect on fungal relative abundance. Glyphosate and fluazifop-p-butyl application resulted in significant increases in fungal relative abundance at the phylum and family level, and increases in pathotroph and saprotroph relative abundance but the application of fluazifop-p-butyl led to decreases in the relative abundance of symbiotrophs. The mechanical treatment also induced declines in the relative abundance of saprotrophs compared to all other treatments.
Treatment differences
Mechanically induced removal/mortality of Ehrharta calycina and Eucalyptus todtiana seedlings in this study was associated with a decline in fungal ASV diversity and ASV richness when compared to no treatment, as shown from assessment after six weeks. This pattern of a reduction in the soil fungal community has been observed in agricultural settings in which soil microbial mass and activity decreased when mechanical removal methods were used (Sun et al. 2020; Zhang et al. 2022). Ascomycete biomass and abundance has also been shown to decrease after mechanical removal of invasive species in both Brazilian Salix lasiolepis woodlands and Chinese Illicium verum plantations (Xiao et al. 2022; Bañuelas et al. 2024). This could be associated with the disruption of fungal hyphal structures and the removal of root substrate, the loss of fungal habitat and carbon exudates creating less suitable conditions for mycorrhizal associations (Hawkins et al. 2023).
There were several significant immediate responses from the fungal community to the herbicides used in our study. These responses may be short-lived and more work as a time series is required to explore these effects. The reduction in symbiotrophs in the fluazifop-p-butyl treatment is a cause for concern, as whilst this reduction in mycorrhizal fungi has been observed in cropping systems (Szwedek-Trzaska and Glowacka 2011; Silveira et al. 2015; Lishchuk et al. 2023), the effects may be much more detrimental for native eucalypts that require symbiotrophic relationships with fungi to enable establishment and growth (Adams et al. 2006; Qin and Yu 2019). The increase in pathogenic fungi observed in this study should be considered when making decisions regarding herbicide use in native ecosystems. Authors have speculated that the increase in pathogens and saprotrophs was due to herbicides reducing plant resistance to pathogens and more dead or weakened material being available to support these fungi. This ‘herbicide-enhanced pathogen’ effect has been observed (Lévesque and Rahe 1992) in, for example, Glycine max seeds swelling with trifluralin application allowing for Fusarium spp. infection (Carson et al. 1991). Another study found that fluazifop-p-butyl, when applied in a sufficiently low concentration (1/10,000th), led to an increase in mycelial dry weight of Aspergillus favus and Cunninghamella echinulata; the former a pathogen of Zea mays and the latter a soil saprotroph (Abdel-Mallek et al. 1996).
The increases in fungal relative abundance we found associated with the glyphosate treatment in the short term are inconsistent with results in the literature. Most observations show fungicidal effects in glasshouse trials, field trials and laboratory culturing (Wolmarans and Swart 2014). Glyphosate applied to pots containing Argentinian Lotus tenuis and Paspalum dilatatum in a glasshouse led to a reduction in mycorrhizal root colonies, even at low rates (0.1 L/ha; Druille et al. 2013). These fungicidal effects were also observed when glyphosate was applied in an Argentine semi-arid grassland, decreasing fungal biomass and species richness (Vázquez et al. 2021). Under laboratory conditions, reduction in growth of Guignardia bidwellii, a root pathogen of Vitus spp., when cultured in agar spiked with 200 g/L glyphosate-based herbicide, was observed (Albrecht and Kortekamp 2009). However in a few cases the opposite has also been shown, with an increase in fungal pathogen abundance after spraying the herbicide, such as increased Fusarium spp. and Pythium spp. infection in Phaseolus vulgaris roots within 12 h of glyphosate application both on plants and pure culture (Meriles et al. 2006). This study may indicate an ability for glyphosate to be degraded by soil fungi before effects are observed. Whilst we did not find commonly-known genera that break glyphosate down (Such as Aspergillius spp. or Fusarium spp.; Castrejón-Godínez et al. 2021) in any significant compositional number, there may be unidentified species from these genera in the dataset or other taxa that can also break glyphosate down. This requires more sequencing of local fungi and identifying the metabolic functions to confirm whether any species can be used in detoxification to aid in considerations of the use of glyphosate.
When comparing the direct and indirect effects of mortality (mechanical vs herbicides) there were enough differences to begin to ascertain an isolated herbicide effect vs an overall plant mortality effect. For example, richness, diversity and abundance of saprotrophs were higher in pots treated with glyphosate- and fluazifop-based herbicides. This is contrary to findings in the wider literature, where either non-significant effects were observed (in cultured media; Dennis et al. 2023) or a decrease in saprotroph richness, diversity and abundance, in both Northwestern US prairie (Roy et al. 2023) and Welsh grasslands (Griffith et al. 2014) treated with fluazifop-p-butyl was observed. However, due to less significant differences between these herbicides and the untreated control, more investigation will be required to confirm the underlying mechanisms.
Species differences
The higher number of symbiotrophs in pots containing Eucalyptus todtiana than the other pot occupants was consistent with findings in the literature; in the soil these fungi require hosts with which to associate. In addition, both Eucalyptus todtiana and Ehrharta calycina have both been observed to form mycorrhizal associations; with ectomycorrhizal fungi and arbuscular mycorrhizal fungi respectively (Albornoz et al. 2017; Tshewang et al. 2022). Ectomycorrhizal fungi far exceed arbuscular mycorrhiza in diversity, especially in Western Australia (Bougher 1995) and associate with Eucalyptus species throughout the lifecycle (Taylor and Alexander 2005; Horton et al. 2013). Arbuscular mycorrhizal fungi are distributed across the globe and associate with a wide diversity of plant species including grasses. However, these have comparatively low diversity and only a few species tend to colonise a single plant at any one time (Barceló et al. 2020; Davison et al. 2020). Furthermore, given that Ehrharta calycina is invasive but Eucalyptus todtiana is native, the native soil used to ‘inoculate’ the pots can reasonably be considered to have contained more mycorrhizal fungi able to associate with native eucalypts over invasive grasses and the invasive grass has very few fungi to associate with in the environment (Birnbaum et al. 2018). The lower number of symbiotrophic fungi on Ehrharta calycina may also result from the ITS primer set used being more appropriate for targeting higher fungi such as ectomycorrhizal species rather than targeting arbuscular mycorrhizal fungi (18S rRNA; Redecker 2000).
The differences in how the soil fungal community responded to the treatments within the pots containing Ehrharta calycina and Eucalyptus todtiana represent an important consideration for the efficacy of herbicide-based invasive species control. The change in fungal community composition in pots containing Ehrharta calycina but not those containing Eucalyptus todtiana could be due to a larger proportion of the fungal community being associated with the rhizosphere of the eucalypts and being able to persist in the root matrix even after the host plant dies (Dos Santos et al. 2023; Rabelo et al. 2023). However, this compositional effect is very weak, with only a small proportion of the variation explained by the model, therefore more investigation will be required. In pots containing both Ehrharta calycina and Eucalyptus todtiana, there were changes in relative abundance under herbicide treatments compared to the controls, with pots containing Ehrharta calycina having eight groups increase in relative abundance (one phylum, six families and two functional groups) and pots containing Eucalyptus todtiana having three groups increase in relative abundance (all phyla) and a decrease in symbiotroph relative abundance. The functional group changes are possibly the most important, as the increases in mean relative abundances of pathotrophs and saprotrophs in pots containing Ehrharta calycina and the decrease in symbiotrophs in pots containing Eucalyptus todtiana could significantly decrease the survival of the dominant host tree through lack of mycorrhizal associations.
Future management
Invasive species are a significant concern for management and conservation of natural areas, particularly in urban woodland fragments (Motard et al. 2011; Dolan 2015). Grassy invasive species will only become more prevalent in areas that are predicted to become drier and warmer, and experience a higher fire frequency, the management of which may include an increase in the use of herbicides globally (Ziska 2020). Public opinion on herbicides has become critical in recent years with many herbicides being banned or phased out (Rawat et al. 2023). The effects of isolated herbicides in this study show that herbicides such as pelargonic acid or fluazifop-p-butyl would not be individually suitable for urban woodland application in this system under the conditions that were tested. We also showed that even if a herbicide is marketed as being specific for particular target, this may not be the case for native plants and the associated fungi as the effects of mortality on the native tree Eucalyptus todtiana and reduction in the relative abundance of symbiotrophic fungi showed. However, when herbicides are used as part of an integrated management strategy, such as in combination with prescribed burns these negative effects on soil fungi seem to be offset (Brace et al. 2025), yet the combination is still just as effective in the management of invasive species (Miller and Miller 2020). Certainly, we indicate the value of incorporating ecological trade-offs on soil fungi revealed by research when making decisions regarding whether to use herbicide in the management of woodland fragments. The patterns observed in this study require further exploration however including, repeat testing under different environmental conditions, to better determine the impact and mode of action of herbicides on below ground organisms, and looking at impacts over longer time periods. The functional changes in soil fungal community observed in this study could further disturb the soil fungal community and complicate subsequent management considerations.
Conclusions
We investigated the short-term (six weeks) ex situ effects of isolated herbicide application on soil fungal communities from Banksia woodlands in south-western Australia. Whilst carried out in a glasshouse environment, this study contributes to the understanding of the effects of individual herbicide application on the fungal component of Banksia woodland soils. The treatment that affected the widest array of soil fungal community metrics was pelargonic acid that was associated with lower total species richness and Shannon diversity (compared to no treatment). Fluazifop-p-butyl treatments resulted in lower relative abundance of symbiotrophs and higher relative abundance of pathotrophs, indicating that for urban woodland application, this herbicide may not be suitable, possibly reducing native species’ establishment and creating a herbicide-enhanced pathogen load in a natural system. Our work contributes to knowledge of specific herbicides and the associated short-term, immediate effects on soil fungal communities that will aid in the future conservation of both above- and below-ground biodiversity.
Data availability
The data that support this study are available as raw molecular data stored at the Sequence Read Archive (SRA) curated by NCBI under the accession number PRJNA1037266.
Declaration of funding
Sites utilised were part of a larger project of ‘Optimising fire management for a resilient future’ (funded by the Australian Research Council Linkage grant LP160100996) with cash contributions provided by the Botanical Gardens and Parks Authority and the Department of Parks and Wildlife (both currently part of Department of Biodiversity, Conservation and Attractions). This work also was funded by in-kind contributions from the School of Science, Edith Cowan University under a higher degree by research scholarship.
Acknowledgements
We are grateful to the Murdoch University and Terrestrial Ecology Research Group (also at Murdoch University) students and volunteers and for the use of their research vehicle to access study sites. We thank Rosie McGuinness for assistance with sample collection. We wish to acknowledge the traditional custodians of the land on which this work was undertaken, the Whadjuk Nyoongar people. We wish to acknowledge and respect their continuing culture and the contribution they make to the life of Perth and the broader region.
References
Abarenkov K, Nilsson RH, Larsson K-H, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T, Sen R, Taylor AFS, Tedersoo L, Ursing Börn M, Vrålstad T, Liimatainen K, Peintner U, Kõljalg U (2010) The UNITE database for molecular identification of fungi – recent updates and future perspectives. New Phytologist 186(2), 281-285.
| Crossref | Google Scholar | PubMed |
Abdel-Mallek A, Abdel-Kader MIA, Omar SA (1996) Effect of the herbicide fluazifop-butyl on fungal populations and activity in soil. Water, Air, and Soil Pollution 86, 151-157.
| Crossref | Google Scholar |
Adams F, Reddell P, Webb MJ, Shipton WA (2006) Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical north-eastern Australia. Australian Journal of Botany 54(3), 271-281.
| Crossref | Google Scholar |
Albornoz FE, Burgess TI, Lambers H, Etchells H, Laliberté E (2017) Native soilborne pathogens equalize differences in competitive ability between plants of contrasting nutrient-acquisition strategies. Journal of Ecology 105(2), 549-557.
| Crossref | Google Scholar |
Albrecht M, Kortekamp A (2009) The in vitro effect of the herbicide Basta® (glufosinate ammonium) on potential fungal grapevine pathogens. European Journal of Horticultural Science 74(3), 112-117.
| Crossref | Google Scholar |
Amundson R (2001) The carbon budget in soils. Annual Review of Earth and Planetary Sciences 29(1), 535-562.
| Crossref | Google Scholar |
Bañuelas DC, Shah NC, Perez JE, Bellier-Igasaki SA, McGauley E, Swanson AC, Arenas A, Treseder KK (2024) Response of ectomycorrhizal fungi to full and selective removal of an invasive tree in riparian woodland. Restoration Ecology 32(7), e14204.
| Crossref | Google Scholar |
Barceló M, Van Bodegom PM, Tedersoo L, den Haan N, Veen GF, Ostonen I, Trimbos K, Soudzilovskaia NA (2020) The abundance of arbuscular mycorrhiza in soils is linked to the total length of roots colonized at ecosystem level. PLoS ONE 15(9), e0237256.
| Crossref | Google Scholar | PubMed |
Birnbaum C, Morald TK, Tibbett M, Bennett RG, Standish RJ (2018) Effect of plant root symbionts on performance of native woody species in competition with an invasive grass in multispecies microcosms. Ecology and Evolution 8(17), 8652-8664.
| Crossref | Google Scholar | PubMed |
Brace AJ, Ruthrof KX, Miller BP, Fontaine JB, Hopkins AJM (2024) Short-term soil fungal community dynamics following fire in Mediterranean climate-type Banksia woodlands. Soil Biology and Biochemistry 199, 109579.
| Crossref | Google Scholar |
Brace AJ, Ruthrof KX, Fontaine JB, Miller BP, Hopkins AJM (2025) Herbicide, not prescribed burning, drives larger shifts in soil fungal communities in a Mediterranean-type urban woodland. Urban Forestry & Urban Greening 105, 128728.
| Crossref | Google Scholar |
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2), 378-400.
| Crossref | Google Scholar |
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nature Methods 13(7), 581-583.
| Crossref | Google Scholar | PubMed |
Carson ML, Arnold WE, Todt PE (1991) Predisposition of soybean seedlings to Fusarium root rot with Trifluralin. Plant Disease 75(4), 342-347.
| Crossref | Google Scholar |
Castrejón-Godínez ML, Tovar-Sánchez E, Valencia-Cuevas L, Rosas-Ramírez ME, Rodríguez A, Mussali-Galante P (2021) Glyphosate pollution treatment and microbial degradation alternatives, a review. Microorganisms 9(11), 2322.
| Crossref | Google Scholar | PubMed |
Chávez-Ortiz P, Tapia-Torres Y, Larsen J, García-Oliva F (2022) Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Applied Soil Ecology 169, 104256.
| Crossref | Google Scholar |
Ciriminna R, Fidalgo A, Ilharco LM, Pagliaro M (2019) Herbicides based on pelargonic acid: herbicides of the bioeconomy. Biofuels, Bioproducts and Biorefining 13(6), 1476-1482.
| Crossref | Google Scholar |
Clements DR, DiTommaso A, Jordan N, Booth BD, Cardina J, Doohan D, Mohler CL, Murphy SD, Swanton CJ (2004) Adaptability of plants invading North American cropland. Agriculture, Ecosystems & Environment 104(3), 379-398.
| Crossref | Google Scholar |
Coleman R, Penner D (2008) Organic acid enhancement of pelargonic acid. Weed Technology 22(1), 38-41.
| Crossref | Google Scholar |
Davison J, García de León D, Zobel M, Moora M, Bueno CG, Barceló M, Gerz M, León D, Meng Y, Pillar VD, Sepp S-K, Soudzilovaskaia NA, Tedersoo L, Vaessen S, Vahter T, Winck B, Öpik M (2020) Plant functional groups associate with distinct arbuscular mycorrhizal fungal communities. New Phytologist 226(4), 1117-1128.
| Crossref | Google Scholar | PubMed |
Dennis PG, Kukulies T, Forstner C, Plisson F, Eaglesham G, Pattison AB (2023) The effects of atrazine, diuron, fluazifop-p-butyl, haloxyfop-p-methyl, and pendimethalin on soil microbial activity and diversity. Applied Microbiology 3(1), 79-89.
| Crossref | Google Scholar |
Dixon P (2003) VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14(6), 927-930.
| Crossref | Google Scholar |
Dolan RW (2015) Changes in plant species composition and structure in two peri-urban nature preserves over 10 years. The American Midland Naturalist 174(1), 33-48.
| Crossref | Google Scholar |
Dos Santos EA, Sabino da Silva-Filho U, Barroso GM, Rabelo JS, De Melo EI, Dos Santos JB (2023) Arbuscular mycorrhizal fungi activity in the rhizosphere of tree seedlings subjected to residual herbicides. Brazilian Journal of Biology 83, e242676.
| Crossref | Google Scholar |
Druille M, Omacini M, Golluscio RA, Cabello MN (2013) Arbuscular mycorrhizal fungi are directly and indirectly affected by glyphosate application. Applied Soil Ecology 72, 143-149.
| Crossref | Google Scholar |
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67(3), 345-366.
| Google Scholar |
Duke SO (2018) The history and current status of glyphosate. Pest Management Science 74(5), 1027-1034.
| Crossref | Google Scholar | PubMed |
Edwards CA, Pimentel D (1989) Impact of herbicides on soil ecosystems. Critical Reviews in Plant Sciences 8(3), 221-257.
| Crossref | Google Scholar |
Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ (2009) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biological Conservation 142(10), 2270-2281.
| Crossref | Google Scholar |
Giglio A, Vommaro ML (2022) Dinitroaniline herbicides: a comprehensive review of toxicity and side effects on animal non-target organisms. Environmental Science and Pollution Research 29(51), 76687-76711.
| Crossref | Google Scholar | PubMed |
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agriculture, Ecosystems & Environment 113(1–4), 17-35.
| Crossref | Google Scholar |
Griffith GW, Graham A, Woods RG, Easton GL, Halbwachs H (2014) Effect of biocides on the fruiting of waxcap fungi. Fungal Ecology 7, 67-69.
| Crossref | Google Scholar |
Grossmann K (2010) Auxin herbicides: current status of mechanism and mode of action. Pest Management Science 66(2), 113-120.
| Crossref | Google Scholar |
Guilherme S, Gaivão I, Santos M, Pacheco M (2012) DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide – elucidation of organ-specificity and the role of oxidative stress. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 743(1–2), 1-9.
| Crossref | Google Scholar |
Hawkins H-J, Cargill RIM, Van Nuland ME, Hagen SC, Field KJ, Sheldrake M, Soudzilovskaia NA, Kiers ET (2023) Mycorrhizal mycelium as a global carbon pool. Current Biology 33(11), R560-R573.
| Crossref | Google Scholar | PubMed |
He B, Hu Y, Wang W, Yan W, Ye Y (2022) The progress towards novel herbicide modes of action and targeted herbicide development. Agronomy 12(11), 2792.
| Crossref | Google Scholar |
Helander M, Saloniemi I, Saikkonen K (2012) Glyphosate in northern ecosystems. Trends in Plant Science 17(10), 569-574.
| Crossref | Google Scholar | PubMed |
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annual Review of Plant Biology 50(1), 473-503.
| Crossref | Google Scholar |
Hidayat I, Preston C (1997) Enhanced metabolism of fluazifop acid in a biotype of Digitaria sanguinalis resistant to the herbicide fluazifop-p-butyl. Pesticide Biochemistry and Physiology 57(2), 137-146.
| Crossref | Google Scholar |
Horton BM, Glen M, Davidson NJ, Ratkowsky D, Close DC, Wardlaw TJ, Mohammed C (2013) Temperate eucalypt forest decline is linked to altered ectomycorrhizal communities mediated by soil chemistry. Forest Ecology and Management 302, 329-337.
| Crossref | Google Scholar |
Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE, Lindahl BD (2012) New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiology Ecology 82(3), 666-677.
| Crossref | Google Scholar | PubMed |
Karpouzas DG, Papadopoulou E, Ipsilantis I, Friedel I, Petric I, Udikovic-Kolic N, Djuric S, Kandeler E, Menkissoglu-Spiroudi U, Martin-Laurent F (2014) Effects of nicosulfuron on the abundance and diversity of arbuscular mycorrhizal fungi used as indicators of pesticide soil microbial toxicity. Ecological Indicators 39, 44-53.
| Crossref | Google Scholar |
Keighery G, Gosper CR, Barrett S, Coates D, Makinson RO (2023) The compounding impacts of disease and weeds after the 2019–20 wildfires on Australian vascular plants and communities. In ‘Australia’s megafires: biodiversity impacts and lessons from 2019–2020’. (Eds L Rumpff, SM Legge, S van Leeuwen, BA Wintle, JCZ Woinarski) p. 243. (CSIRO Publishing)
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15(3), 259-263.
| Crossref | Google Scholar |
Kudsk P (2008) Optimising herbicide dose: a straightforward approach to reduce the risk of side effects of herbicides. The Environmentalist 28, 49-55.
| Crossref | Google Scholar |
Laiho R, Prescott CE (1999) The contribution of coarse woody debris to carbon, nitrogen, and phosphorus cycles in three Rocky Mountain coniferous forests. Canadian Journal of Forest Research 29(10), 1592-1603.
| Crossref | Google Scholar |
Leino L, Tall T, Helander M, Saloniemi I, Saikkonen K, Ruuskanen S, Puigbo P (2021) Classification of the glyphosate target enzyme (5-enolpyruvylshikimate-3-phosphate synthase) for assessing sensitivity of organisms to the herbicide. Journal of Hazardous Materials 408, 124556.
| Crossref | Google Scholar | PubMed |
Lekberg Y, Wagner V, Rummel A, McLeod M, Ramsey PW (2017) Strong indirect herbicide effects on mycorrhizal associations through plant community shifts and secondary invasions. Ecological Applications 27(8), 2359-2368.
| Crossref | Google Scholar | PubMed |
Lévesque CA, Rahe JE (1992) Herbicide interactions with fungal root pathogens, with special reference to glyphosate. Annual Review of Phytopathology 30(1), 579-602.
| Crossref | Google Scholar |
Li X, Miao W, Gong C, Jiang H, Ma W, Zhu S (2013) Effects of prometryn and acetochlor on arbuscular mycorrhizal fungi and symbiotic system. Letters in Applied Microbiology 57(2), 122-128.
| Crossref | Google Scholar | PubMed |
Lin H, Peddada SD (2020) Analysis of compositions of microbiomes with bias correction. Nature Communications 11(1), 3514.
| Crossref | Google Scholar | PubMed |
Lin H, Peddada SD, Lin MH (2021) Package ‘ANCOMBC’. Available at https://github.com/FrederickHuangLin/ANCOMBC
Lindsey III BE, Rivero L, Calhoun CS, Grotewold E, Brkljacic J (2017) Standardized method for high-throughput sterilization of Arabidopsis seeds. JoVE: Journal of Visualized Experiments 128, e56587.
| Crossref | Google Scholar |
Lishchuk A, Parfenyk A, Horodyska І, Boroday V, Ternovyi Y, Tymoshenko L (2023) Environmental risks of the pesticide use in agrocenoses and their management. Journal of Ecological Engineering 24(3), 199-212.
| Crossref | Google Scholar |
Loddo D, Jagarapu KK, Strati E, Trespidi G, Nikolić N, Masin R, Berti A, Otto S (2023) Assessing herbicide efficacy of pelargonic acid on several weed species. Agronomy 13(6), 1511.
| Crossref | Google Scholar |
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17(1), 10-12.
| Crossref | Google Scholar |
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4), e61217.
| Crossref | Google Scholar | PubMed |
Meriles JM, Gil SV, Haro RJ, March GJ, Guzmán CA (2006) Glyphosate and previous crop residue effect on deleterious and beneficial soil-borne fungi from a peanut–corn–soybean rotations. Journal of Phytopathology 154(5), 309-316.
| Crossref | Google Scholar |
Miller RG, Miller BP (2020) Adding fuel to the fire: invasive grass management and fire: Exploring grassy weeds to reduce bushfire risk. Landscope 36. Available at https://library.dbca.wa.gov.au/static/Journals/080052/080052-36.009.pdf
Motard E, Muratet A, Clair-Maczulajtys D, Machon N (2011) Does the invasive species Ailanthus altissima threaten floristic diversity of temperate peri-urban forests? Comptes Rendus. Biologies 334(12), 872-879.
| Crossref | Google Scholar | PubMed |
Muñoz M, Torres-Pagán N, Jouini A, Araniti F, Sánchez-Moreiras AM, Verdeguer M (2022) Control of problematic weeds in Mediterranean vineyards with the bioherbicide pelargonic acid. Agronomy 12(10), 2476.
| Crossref | Google Scholar |
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology 20, 241-248.
| Crossref | Google Scholar |
Oksanen J, Simpson G, Lahti L, et al. (2017) Vegan: Community Ecology Package. R Package Version 2.4. 4 (2017). Available at https://github.com/vegandevs/vegan
Paini DR, Sheppard AW, Cook DC, De Barro PJ, Worner SP, Thomas MB (2016) Global threat to agriculture from invasive species. Proceedings of the National Academy of Sciences 113(27), 7575-7579.
| Crossref | Google Scholar |
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11(5), 1633-1644.
| Crossref | Google Scholar |
Pranaswi D, Jagtap MP, Asewar BV, Gokhale DN, Shinde GU (2022) Weed control efficiency with herbicide application by the combination of drone and knapsack sprayer in wheat (Triticum aestivum L.). The Pharma Innovation Journal 11(1), 741-744.
| Google Scholar |
Pratt JR, Melendez AE, Barreiro R, Bowers NJ (1997) Predicting the ecological effects of herbicides. Ecological Applications 7(4), 1117-1124.
| Crossref | Google Scholar |
Qin F, Yu S (2019) Arbuscular mycorrhizal fungi protect native woody species from novel weapons. Plant and Soil 440, 39-52.
| Crossref | Google Scholar |
R Studio Team (2021) RStudio: integrated development environment for R. RStudio Inc., Boston MA. Available at http://www.rstudio.com/
Rabelo JS, Dos Santos EA, de Melo EI, Vaz MGMV, de Oliveira Mendes G (2023) Tolerance of microorganisms to residual herbicides found in eucalyptus plantations. Chemosphere 329, 138630.
| Crossref | Google Scholar | PubMed |
Rawat D, Bains A, Chawla P, Kaushik R, Yadav R, Kumar A, Sridhar K, Sharma M (2023) Hazardous impacts of glyphosate on human and environment health: occurrence and detection in food. Chemosphere 329, 138676.
| Crossref | Google Scholar |
Redecker D (2000) Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10(2), 73-80.
| Crossref | Google Scholar |
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecological Applications 15(2), 618-627.
| Crossref | Google Scholar |
Ritchie AL, Stevens JC, Erickson TE (2020) Developing extruded seed pellets to overcome soil hydrophobicity and seedling emergence barriers. Ecological Solutions and Evidence 1(2), e12024.
| Crossref | Google Scholar |
Ritchie AL, Svejcar LN, Ayre BM, Bolleter J, Brace A, Craig MD, Davis B, Davis RA, Van Etten EJB, Fontaine JB, Fowler WM, Froend RH, Groom C, Hardy GESJ, Hooper P, Hopkins AJM, Hughes M, Krauss SL, Leopold M, Miller BP, Miller RG, Ramalho CE, Ruthrof KX, Shaw C, Stevens JC, Tangney R, Valentine LE, Veneklaas EJ, Hobbs RJ (2021) Corrigendum to: a threatened ecological community: research advances and priorities for Banksia woodlands. Australian Journal of Botany 69(2), 111.
| Crossref | Google Scholar |
Roca E, D’Errico E, Izzo A, Strumia S, Esposito A, Fiorentino A (2009) In vitro saprotrophic basidiomycetes tolerance to pendimethalin. International Biodeterioration & Biodegradation 63(2), 182-186.
| Crossref | Google Scholar |
Rose MT, Cavagnaro TR, Scanlan CA, Rose TJ, Vancov T, Kimber S, Kennedy IR, Kookana RS, Van Zwieten L (2016) Impact of herbicides on soil biology and function. Advances in Agronomy 136, 133-220.
| Crossref | Google Scholar |
Roy BA, Hamman ST, Soukup H, Messinger W, Vandegrift R, Blount K, Giles DEL, Kaye TN (2023) Consequences of fire and other prairie management treatments for macrofungi in the Pacific Northwest of the USA. Fungal Ecology 65, 101279.
| Crossref | Google Scholar |
Rüegg WT, Quadranti M, Zoschke A (2007) Herbicide research and development: challenges and opportunities. Weed Research 47(4), 271-275.
| Crossref | Google Scholar |
Russell C, Schultz CB (2010) Effects of grass-specific herbicides on butterflies: an experimental investigation to advance conservation efforts. Journal of Insect Conservation 14(1), 53-63.
| Crossref | Google Scholar |
Sanyal D, Shrestha A (2008) Direct effect of herbicides on plant pathogens and disease development in various cropping systems. Weed Science 56(1), 155-160.
| Crossref | Google Scholar |
Schnoor TK, Lekberg Y, Rosendahl S, Olsson PA (2011) Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 21(3), 211-220.
| Crossref | Google Scholar | PubMed |
Silveira HM, Silva DV, Melo CAD, Neto MDC, Saraiva DT, Ferreira EA, Silva AA, Freitas MS (2015) Mycorrhizal association and microbial activity of soil cultivated with cassava after application of mesotrione and fluazifop-p-butyl. Planta Daninha 33(2), 275-281.
| Crossref | Google Scholar |
Smith JR, Ferry BW (1979) The effects of simazine, applied for weed control, on the mycorrhizal development of Pinus seedlings. Annals of Botany 43(1), 93-99.
| Crossref | Google Scholar |
Sun B, Chen X, Zhang X, Liang A, Whalen JK, McLaughlin NB (2020) Greater fungal and bacterial biomass in soil large macropores under no-tillage than mouldboard ploughing. European Journal of Soil Biology 97, 103155.
| Crossref | Google Scholar |
Swift D, Cresswell K, Johnson R, Stilianoudakis S, Wei X (2023) A review of normalization and differential abundance methods for microbiome counts data. WIREs Computational Statistics 15(1), e1586.
| Crossref | Google Scholar |
Szwedek-Trzaska A, Glowacka A (2011) Seeking ways to eradicate potentially pathogenic fungi isolated from soil. Polish Journal of Environmental Studies 20(5), 1313-1318.
| Google Scholar |
Taylor AFS, Alexander I (2005) The ectomycorrhizal symbiosis: life in the real world. Mycologist 19(3), 102-112.
| Crossref | Google Scholar |
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Põldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Pärtel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson K-H, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo L-D, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K (2014) Global diversity and geography of soil fungi. Science 346(6213), 1256688.
| Crossref | Google Scholar | PubMed |
Tshewang S, Rengel Z, Siddique KHM, Solaiman ZM (2022) Microbial consortium inoculant increases pasture grasses yield in low-phosphorus soil by influencing root morphology, rhizosphere carboxylate exudation and mycorrhizal colonisation. Journal of the Science of Food and Agriculture 102(2), 540-549.
| Crossref | Google Scholar | PubMed |
Uhlig S (1966) Uber den einfluss von chlor-bis-athylamino-s-triazin (Simazin) auf die bildung ektotropher Mykorrhiza bei Picea abies und Pinus sylvestris. Arch Forstw 15, 463-464.
| Google Scholar |
Van Bruggen AHC, He MM, Shin K, Mai V, Jeong K, Finckh MR, Morris JG, Jr (2018) Environmental and health effects of the herbicide glyphosate. Science of the Total Environment 616-617, 255-268.
| Crossref | Google Scholar | PubMed |
Vázquez MB, Moreno MV, Amodeo MR, Bianchinotti MV (2021) Effects of glyphosate on soil fungal communities: a field study. Revista Argentina de Microbiología 53(4), 349-358.
| Crossref | Google Scholar | PubMed |
Walker KA, Ridley SM, Lewis T, Harwood JL (1988) Fluazifop, a grass-selective herbicide which inhibits acetyl-CoA carboxylase in sensitive plant species. Biochemical Journal 254(1), 307-310.
| Crossref | Google Scholar | PubMed |
Wickham H (2011) ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics 3(2), 180-185.
| Crossref | Google Scholar |
Wilkinson SP, Davy SK, Bunce M, Stat M (2018) Taxonomic identification of environmental DNA with informatic sequence classification trees. PeerJ Preprints 6, e26812v1.
| Crossref | Google Scholar |
Wolmarans K, Swart WJ (2014) Influence of glyphosate, other herbicides and genetically modified herbicide-resistant crops on soil microbiota: a review. South African Journal of Plant and Soil 31(4), 177-186.
| Crossref | Google Scholar |
Xiao J, Chen S, Sun Y, Wu S, Liang W, Yang S (2022) Effects of mechanical weeding on soil fertility and microbial community structure in star anise (Illicium verum Hook. f.) plantations. PLoS ONE 17(4), e0266949.
| Crossref | Google Scholar |
Yates RJ, Howieson JG, Hungria M, Bala A, O’Hara GW, Terpolilli J (2016) Authentication of rhizobia and assessment of the legume symbiosis in controlled plant growth systems. In ‘Working with rhizobia’. (Eds JG Howieson, MJ Dilworth) pp. 73–108. (Australian Centre for International Agricultural Research)
Zaller JG, Heigl F, Ruess L, Grabmaier A (2014) Glyphosate herbicide affects belowground interactions between earthworms and symbiotic mycorrhizal fungi in a model ecosystem. Scientific Reports 4, 5634.
| Crossref | Google Scholar | PubMed |
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences 23(5), 431-452.
| Crossref | Google Scholar |
Zhang Y, Bo G, Shen M, Shen G, Yang J, Dong S, Shu Z, Wang Z (2022) Differences in microbial diversity and environmental factors in ploughing-treated tobacco soil. Frontiers in Microbiology 13, 924137.
| Crossref | Google Scholar | PubMed |
Zhao J, Wang X, Shao Y, Xu G, Fu S (2011) Effects of vegetation removal on soil properties and decomposer organisms. Soil Biology and Biochemistry 43(5), 954-960.
| Crossref | Google Scholar |
Ziska LH (2020) Climate change and the herbicide paradigm: visiting the future. Agronomy 10(12), 1953.
| Crossref | Google Scholar |


